A carbohydrate is a biological molecule consisting of carbon (C),hydrogen (H) and oxygen (O) atoms,
Carbohydrate Function
 Carbohydrates perform numerous roles in living organisms. They can be the source of energy (glucose) or Polysaccharides can serve for the storage of energy (e.g., starch and glycogen) or as structural components (e.g., cellulose in plants and chitin in arthropods).
Carbohydrates perform numerous roles in living organisms. They can be the source of energy (glucose) or Polysaccharides can serve for the storage of energy (e.g., starch and glycogen) or as structural components (e.g., cellulose in plants and chitin in arthropods).How do carbohydrates enter the food chain?
Simple Sugars
Saccharide Classification
The saccharides are divided into four chemical groups: monosaccharides,disaccharides, oligosaccharides, and polysaccharides. In general, the monosaccharides and disaccharides, which are smaller (lower molecular weight) carbohydrates, are commonly referred to as sugars.[6] The word saccharide comes from the Greekword σάκχαρον (sákkharon), meaning "sugar." While the scientific nomenclature of carbohydrates is complex, the names of the monosaccharides and disaccharides very often end in the suffix -ose. For example, grape sugar is the monosaccharide glucose, cane sugar is the disaccharide sucrose and milk sugar is the disaccharide lactose.
Disaccharides
Monosaccharides are joined together with a 1-4 glycosidic bond. A condensation reaction of course!
Different monosaccharides bonded together form different disaccharides.
Watch this summary video.
Functions are diverse and determined by what monosaccharides bond together.
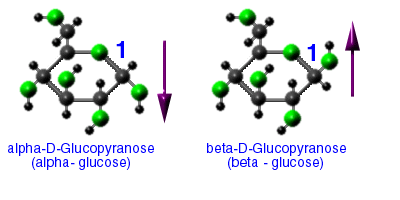
There are two stereo isomers of glucose
These play a huge role in determining the functions of the polysaccharides we will be looking at.
Starch
When many molecules of alpha-glucose are joined together, starch is formed.
Different Forms of Starch.
Cellulose
Condensation of many beta-glucose molecules. In order for 1-4 glycosidic bonds to form between adjacent OH groups - every second beta glucose needs to flip 180 degrees (upside down) so the OH groups are next to each other.
Testing for reducing or non-reducing sugars.
To understand how we can tell, you need to know what a reducing agent.
A reducing agent (also called a reductant or reducer) is an element or compound that loses (or "donates") an electron to another chemical species in a redox chemical reaction. Since the reducing agent is losing electrons, it is said to have been oxidized.
A reducing sugar is any sugar that is capable of acting as a reducing agent because it has a free aldehyde group or a free ketone group. All monosaccharides are reducing sugars, along with some disaccharides, oligosaccharides, and polysaccharides.
The Relevance to the Benedicts Test.
2+ +
Cu-------reducing sugars add an electron-------> Cu
ALL MONOSACCHARIDES ARE REDUCING SUGARS
So monosaccharides will always reduce the Blue Copper 2+
ion into the Brick red Copper + ion.
SOME DISACCHARIDES ARE REDUCING SUGARS.
eg So maltose will always reduce the Blue Copper
2+ ion into the Brick red Copper + ion but sucrose doesn't.
Here is an excellent video showing how to quantify reducing sugars using a colorimeter.












No comments:
Post a Comment