Enzymes are biological catalysts that can speed up reactions without making a difference to the reaction.
Lets revise what you learnt in GCSE!
Enzymes are specific - they only work on one substrate/reaction.
- Enzymes are Biological Catalysts. They increase the rate of Metabolic reactions. Almost all Biological Reactions involve Enzymes. All enzymes are Globular Proteins with a specific Tertiary Shape. They are usually specific to only one reaction.
- The part of the Enzyme that acts a Catalyst is called the Active Site. The rest of the Enzyme is much larger and is involved in maintaining the specific shape of of the Enzyme.
- When a reaction involving an Enzyme occurs, a Substrate is turned into aProduct. The Substrate can be one or more molecules. The Active Site of an Enzyme is Complementary to the Substrate it catalyses.
 |
| Click here to see this animation |
Enzymes are very important in metabolic pathways, different enzymes catalysing products that then become substrates in the next step and so on.
Below: E = enzyme, P = product/substrate
The reaction below shows the transformation of Po into P4 through three different reactions - each step is catalysed by a different enzyme.
There are two types of reactions.
Catabolic - metabolites are broken down into smaller molecules. Energy is released.
Anabolic reactions - energy is used to combine smaller molecules into larger ones.
Many important metabolic reactions are catalysed by many enzymes.
eg: the citric acid cycle in aerobic respiration. Remember 'ase' at the end of a name, indicates an enzyme. These enzymes indicate what their jobs are.
Examples of enzymes.
Catalase - an intracellular enzyme.
Hydrogen peroxide is a harmful by product of many normal metabolic processes; to prevent damage to cells and tissues, it must be quickly converted into other, less dangerous substances. To this end, catalase is frequently used by cells to rapidly catalyse the decomposition of hydrogen peroxide into less-reactive gaseous oxygen and water molecules


Extracellular enzymes
Amylase -
An amylase is an enzyme that catalyses the hydrolysis of starch into sugars. Amylase is present in the saliva of humans and some other mammals, where it begins the chemical process of digestion. Foods that contain large amounts of starch but little sugar, such as rice and potatoes, may acquire a slightly sweet taste as they are chewed because amylase degrades some of their starch into sugar. The pancreas and salivary gland make amylase (alpha amylase) to hydrolyse dietary starch into disaccharides and trisaccharides which are converted by other enzymes to glucose to supply the body with energy. Plants and some bacteria also produce amylase.

Trypsin, a protease found in the digestive system of many vertebrates, where it hydrolyses proteins. Trypsin cleaves peptide chains.

A cofactor is a substance that has to be present to ensure that an enzyme catalysed reaction takes place. In other words - some enzymes need help from other non-protein molecules.
Prosthetic groups - part of the enzymatic structure. A cofactor that is permanently bound by covalent bonds to the enzyme.
eg; the zinc ion in the enzyme carbonic anhydrase.
The blue circle represents the permanently bound zinc ion.
The carbonic anhydrase enables carbon dioxide to be carried in the red blood cells. This reaction can work forwards or in reverse.
Some cofactors are not permanently bound to the enzymes that they are associated with.
These ions make the enzyme/substrate complex form more easily - therefore the enzyme/substrate complex forms more often in the presence of the cofactor. These are not permanently bound to the enzyme.
eg: amylase will only function if chloride is present - but the chloride is not permanently bound.
Coenzymes are organic molecules that are required by certain enzymes to carry out catalysis. They bind to the active site of the enzyme and participate in catalysis but are not considered substrates of the reaction.
This explains why vitamin and mineral deficiencies have such devastating effects.
The Mechanism of Enzyme Action
Lock and Key hypothesis -
The enzyme has an active site at the exact right shape for the substrates.
The Induced Fit model
The enzyme has a different shape to the substrate - but upon binding, the enzyme changes shape so it can become the correct shape to catalyse the reaction.
A couple of videos to help you understand.
Enzymes work by lowering activation energy.
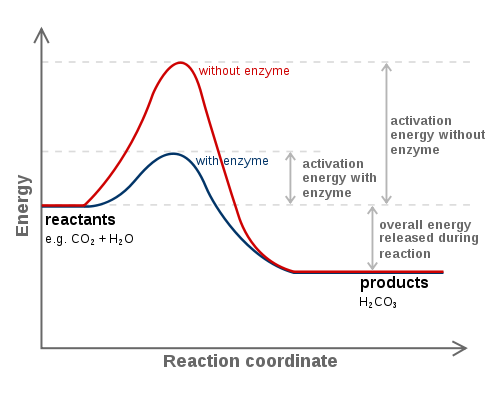 |
Click here to see an excellent animation on how enzymes lower activation energy and what activation energy.
General Collision theory
how to speed up chemical reactions.
Now with a bit more of a conventional twist....
How can we affect the reaction rate of enzymes using this chemical knowledge?
Temperature
Enzymes work best at an optimum temperature.
- Below this, an increase in temperature provides more kinetic energy to the molecules involved. The numbers of collisions between enzyme and substrate will increase so the rate will too.
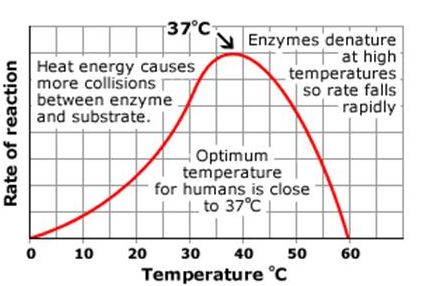
- Above the optimum temperature, and the enzymes are denatured. Bonds holding the structure together will be broken, the active site loses its shape and will no longer work. This does not happen to all enzymes all at once. In the graph above, at 45 degrees, some of the enzymes denature meaning fewer are available to catalyse the reaction so the reaction rate decreases. As the temperature increases, more enzymes denature and so fewer have functional active sites and they cannot catalyse the reaction. At 60 degrees, all enzymes have denatured so there are none available to catalyse the reaction. There is now no reaction taking place.

pH
It ranges from pH1 to pH14. Lower pH values mean higher H+ concentrations and lower OH- concentrations.
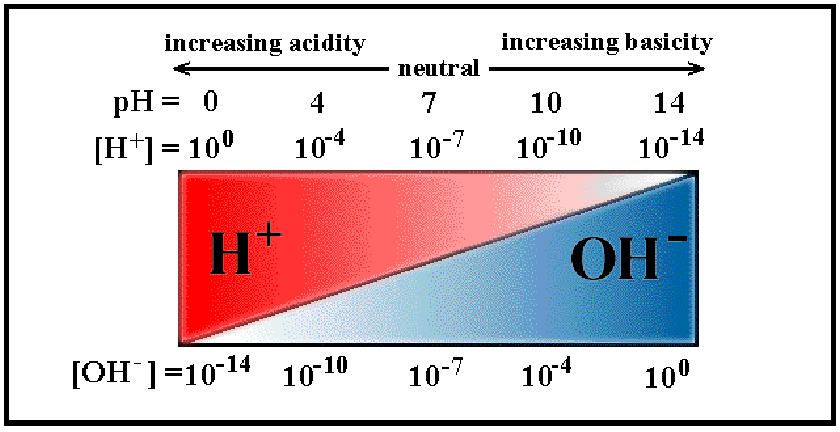
Acid solutions have pH values below 7, and Basic solutions (alkalis are bases) have pH values above 7. Deionised water is pH7, which is termed 'neutral'.
H+ and OH- Ions are charged and therefore interfere with Hydrogen and Ionic bonds that hold together an enzyme, since they will be attracted or repelled by the charges created by the bonds. This interference causes a change in shape of the enzyme, and importantly, its Active Site.

Different enzymes have different Optimum pH values. This is the pH value at which the bonds within them are influenced by H+ and OH- Ions in such a way that the shape of their Active Site is the most Complementary to the shape of their Substrate. At the Optimum pH, the rate of reaction is at an optimum.
Any change in pH above or below the Optimum will quickly cause a decrease in the rate of reaction, since more of the enzyme molecules will have Active Sites whose shapes are not (or at least are less) Complementary to the shape of their Substrate.

Small changes in pH above or below the Optimum do not cause apermanent change to the enzyme, since the bonds can be reformed. However, extreme changes in pH can cause enzymes to Denature and permanently lose their function.
Enzymes in different locations have different Optimum pH values since their environmental conditions may be different. For example, the enzyme Pepsin functions best at around pH2 and is found in the stomach, which contains Hydrochloric Acid (pH2).
Changing concentrations of substrates and enzymes
Changing the concentration of a substance only affects the rate of reaction if it is the limiting factor: that is, it the factor that is stopping a reaction from preceding at a higher rate.
If it is the limiting factor, increasing concentration will increase the rate of reaction up to a point, after which any increase will not affect the rate of reaction. This is because it will no longer be the limiting factor and another factor will be limiting the maximum rate of reaction.
As a reaction proceeds, the rate of reaction will decrease, since the Substrate will get used up. The highest rate of reaction, known as the Initial Reaction Rate is the maximum reaction rate for an enzyme in an experimental situation.
Substrate Concentration

For an enzyme-catalysed reaction, there is usually a hyperbolic relationship between the rate of reaction and the concentration of substrate, as shown below:
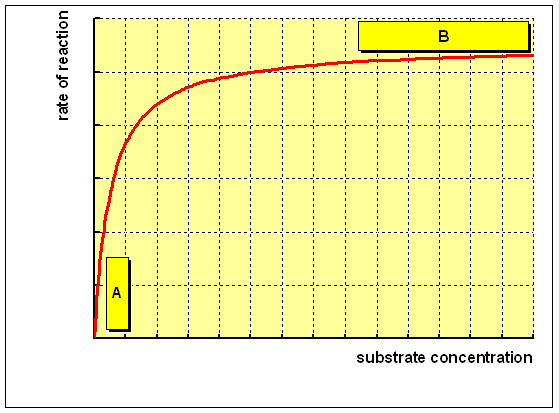
(A) At low concentration of substrate, there is a steep increase in the rate of reaction with increasing substrate concentration. The catalytic site of the enzyme is empty, waiting for substrate to bind, for much of the time, and the rate at which product can be formed is limited by the concentration of substrate which is available.
(B) As the concentration of substrate increases, the enzyme becomes saturated with substrate. As soon as the catalytic site is empty, more substrate is available to bind and undergo reaction. The rate of formation of product now depends on the activity of the enzyme itself, and adding more substrate will not affect the rate of the reaction to any significant effect.

Enzyme concentration
What does rate mean and how do we work it out?
When experimentally determining how fast a reaction is occurring, we time how long it takes using seconds. If a reaction takes more time to complete, it's obviously a slower reaction. If a reaction takes less time to complete, then it's a fast reaction. We can convert these numbers into rate by using the formula
Rate = 1/t (where t is time is seconds)
1/t just gives a quantitative value to comparing the rates of reaction.
i.e. if a reaction finishes in 1 second, then the rate = 1
if a reaction finishes in 3 seconds, then the rate = 1/3
Obviously the one that finished in less time is quicker, 3 times quicker, which is shown by 1/t.
Try it yourself!
Here are a couple of virtual labs to see what the effect pH, temperature and substrate concentration has on the rate of reaction of enzymes.
First click here to visit an excellent site showing how changing different parameters can alter the rate of enzyme reactions.
First click here to visit an excellent site showing how changing different parameters can alter the rate of enzyme reactions.
 |
| Click here to go to the virtual lab |
Information for this page was sourced from
http://alevelnotes.com/Factors-affecting-Enzyme-Activity


















An enzyme is a protein or RNA produced by living cells, which is highly specific and highly catalytic to its substrates. Enzymes are a very important type of macromolecular biological catalysts. Due to the action of enzymes, chemical reactions in organisms can also be carried out efficiently and specifically under mild conditions. enzyme definition and classification
ReplyDelete