Why do we eat food?
We eat food to get the building blocks for making molecules our body needs, to get minerals to enable reactions to occur and to get energy via respiration.
Metabolism: all chemical reactions, catabolic and anabolic, that occur within an organism.

Respiration: a process in living organisms involving the release of energy from food.

Click on the Magazine to get your free informative FOOD issue of Big Picture.
Molecular Bonding
REVISION:
You need to know TWO types of chemical bonding from your GCSE Chemistry days.
IONIC BONDING
Ionic bonding occurs between positive and negative ions, which attract each other and bind together to form ionic compounds.
For example, lithium chloride consists of Li+ ions and Cl- ions bound together.
For example, lithium chloride consists of Li+ ions and Cl- ions bound together.
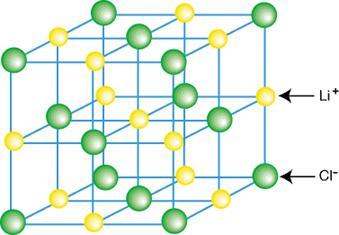
Each ion is surrounded by oppositely charged ions held in place by electrostatic attraction and forming an ionic crystal lattice. The ions in a crystal lattice are very strongly bonded - a high temperature is required to melt the crystal.
Where do the ions come from?
Metal ions
In some circumstances metal atoms may lose electrons. The atom then has more protons than electrons and so it will be positively charged, a positive ion.




.
Non-metal ions


Non-metal atoms may gain electrons and become negatively charged.
Example: An oxygen atom may gain two electrons and become an O2- ion.
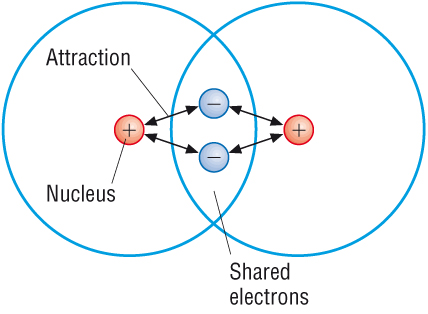
COVALENT BONDING
A covalent bond is formed between non metal atoms, which combine together by sharing electrons. Covalent compounds have no free electrons and no ions so they don't conduct electricity.
END OF REVISION SECTION
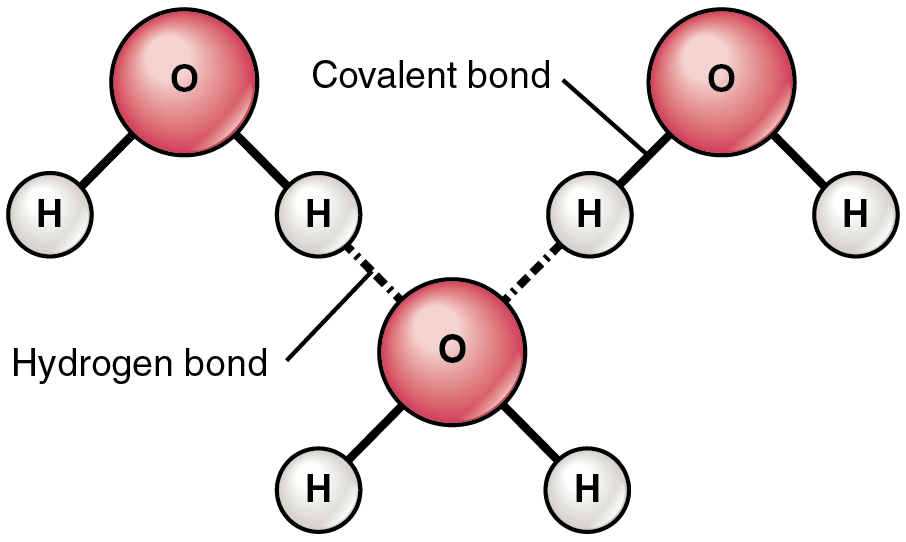
You also need to understand how HYDROGEN bonds are formed - these are intermolecular bonds very common in biological structures.

Carbon - a very versatile atom!


Most biological molecules are made up of many smaller molecules.

FOR EXAMPLE
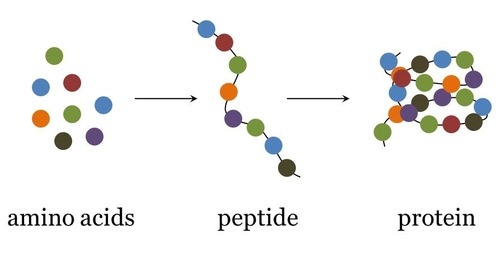

Putting monomers together - condensation.

Breaking polymers down into monomers - hydrolysis
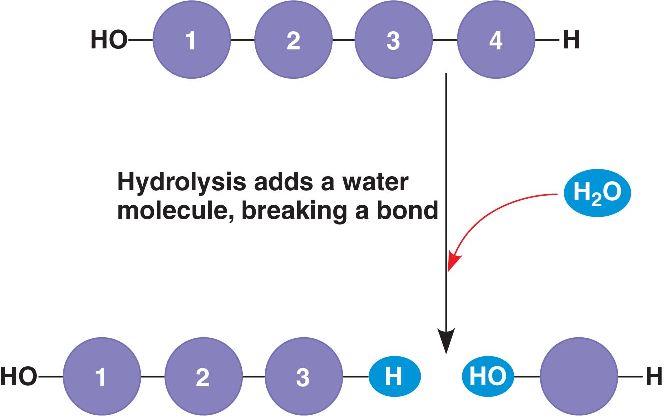
More details on the following molecules can be found by clicking the links below.
The biological properties of Water


No comments:
Post a Comment